Clinical Trial Management Solutions
Empowering Excellence in Clinical Research
At Diamind Solutions, we deliver innovative, comprehensive Clinical Trial Management Solutions designed to simplify operations, enhance data quality, and ensure compliance across all aspects of clinical research. With a suite of integrated modules, our platform enables seamless management of every trial phase, empowering sponsors, researchers, and site teams to achieve excellence.
Core Capabilities of Our Clinical Trial Management Solutions
1. Randomization (IRT):
Ensure precise and scientifically robust treatment allocation with advanced randomization algorithms. Our platform supports adaptive and non-adaptive randomization, balancing across unlimited covariates, multi-arm designs, and double-blinded protocols.
2. eConsent:
Streamline patient enrollment with electronic consent capabilities. Our eConsent module supports multiple workflows, including informed, surrogate, and emergency consent, reducing enrollment time while ensuring compliance and patient comprehension.
3. Drug/Device/IP Management:
Manage investigational products with ease using integrated inventory tracking, drug allocation workflows, and cold chain monitoring. Our platform automates drug assignment during randomization and ensures full compliance with regulatory standards.
4. Bio-sample/Specimen Tracking:
Track biological samples throughout the trial lifecycle with tools for kit creation, real-time tracking, and secure data integration. This module ensures specimen integrity and supports compliance with global regulations.
5. Patient Visit/Event Management:
Organize and monitor patient visits and milestones with automated scheduling and real-time tracking. Seamlessly integrate with other trial workflows to reduce errors and improve visit adherence.
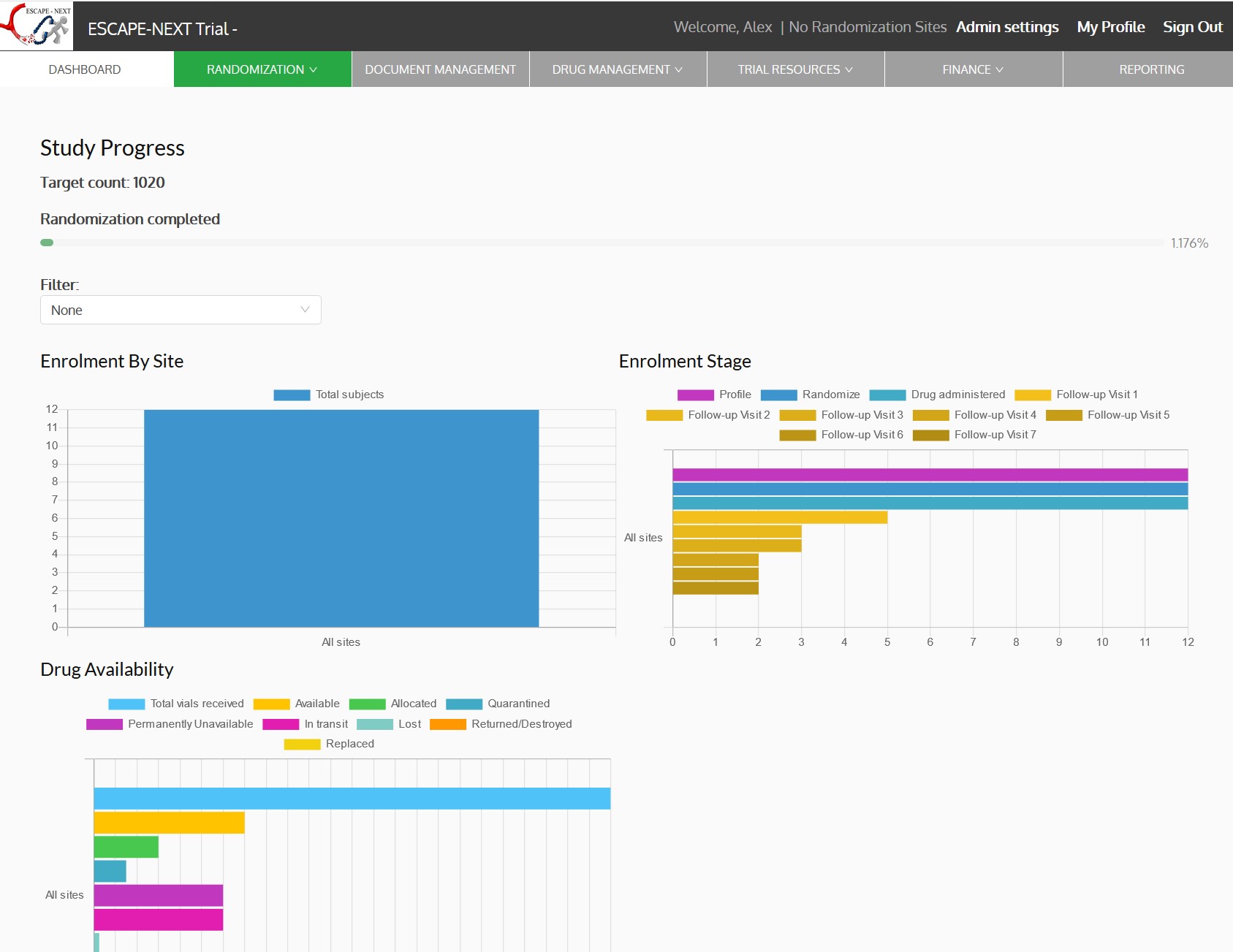
6. Dashboards, Reporting & Notifications:
Gain actionable insights with customizable dashboards and comprehensive reporting tools. Monitor key metrics like enrollment rates, visit completion, and drug availability while automating notifications to keep stakeholders informed.
7. Finance Management:
Simplify budgeting and payments with milestone-linked financial workflows. Automate invoicing and track payments across multi-site trials, ensuring accuracy and transparency.
8. Delegation Log Management:
Maintain compliant delegation logs with role-based task assignments and electronic signatures. Manage updates and revisions seamlessly while tracking responsibilities at the site and trial levels.
9. Electronic Trial Master File (eTMF) and Remote Monitoring:
Store, organize, and monitor trial documents with a secure eTMF. Enable remote monitoring with real-time access to site records, reducing the need for in-person visits.
10. CRF/Form Builder:
Design and deploy custom data collection forms with ease. Capture critical data accurately and integrate it seamlessly across trial workflows.
11. Patient Portal:
Empower participants with secure access to study information, visit schedules, and eConsent documents. Enhance engagement with real-time notifications and easy data entry for patient-reported outcomes.
12. Pre-Built Data Integrations:
Ensure seamless interoperability with industry-leading platforms like REDCap, DFDiscover, and EHR systems. Automate data exchange to eliminate manual errors and improve efficiency.
13. Simulation Management:
Test and refine trial designs before implementation with advanced simulation tools. Evaluate randomization strategies, balancing variables, and sample sizes to optimize trial outcomes.
14. Role-Based Access Controls:
Secure your trial data with granular access controls, ensuring users only access the information and functions relevant to their roles. Maintain transparency with comprehensive audit trails.
Why Choose Our Clinical Trial Management Solutions?
- Integrated Platform: Manage every aspect of your clinical trial from a single, unified system.
- Compliance-Driven Design: Ensure adherence to global standards like FDA 21 CFR Part 11, HIPAA, GDPR, and GCP.
- Scalable for Any Trial Size: From single-site studies to multi-center, global trials, our solutions adapt to your needs.
- Real-Time Insights: Make informed decisions with actionable dashboards, real-time data, and automated workflows.
- Streamlined Workflows: Eliminate redundant tasks, reduce errors, and save time with seamless integration between modules.
Transform Your Clinical Trials
Contact us today to learn how our Clinical Trial Management Solutions can optimize your trial operations, improve data quality, and ensure regulatory compliance.
Ready to enroll?Get started today!
Our main areas of expertise are in the Healthcare, Academic, Education as well as the Energy Sector, where we provide solutions for a number of needs:
- - Clinical Trial Management Solutions (CTMS)
- - Interactive Response Technologies (IRT)
- - Randomization & Drug Management
- - Document Management & eTMF
- - eConsent
- - EHR Integrations
- - Custom Application Development
- - Business Intelligence & Data Integration