Our Projects
Depending on your needs and business plan, this may be a Clinical Trial Management Solution, a Custom Mobile Application, a Business Intelligence & Analytics Solution, or even all of the above. We will meet with you, understand your needs, and then try to find the solution that offers the best fit for your needs (by analyzing cost, technology, complexity, or other factors).
Success Stories
The solutions has been used in various trials, such as:
Clinical Trial / Custom Application
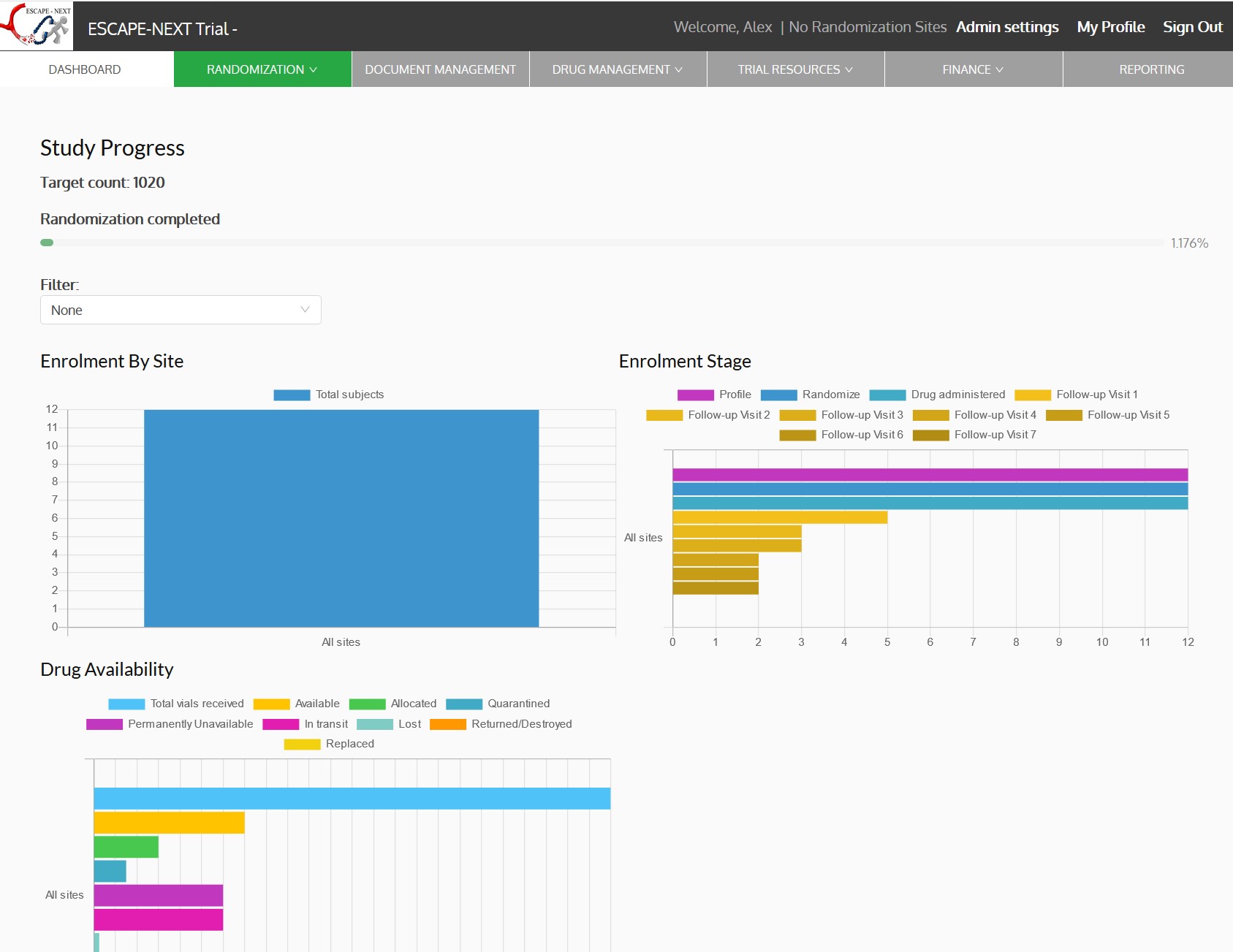
ESCAPE-NEXT Trial
ESCAPE-NEXT is a multicentre, randomized, double-blinded, placebo-controlled, parallel Group, singledose trial designed to determine the efficacy and safety of nerinetide in participants with acute ischemic stroke undergoing endovascular thrombectomy excluding thrombolysis.
Read Use CaseClinical Trial / Custom Application
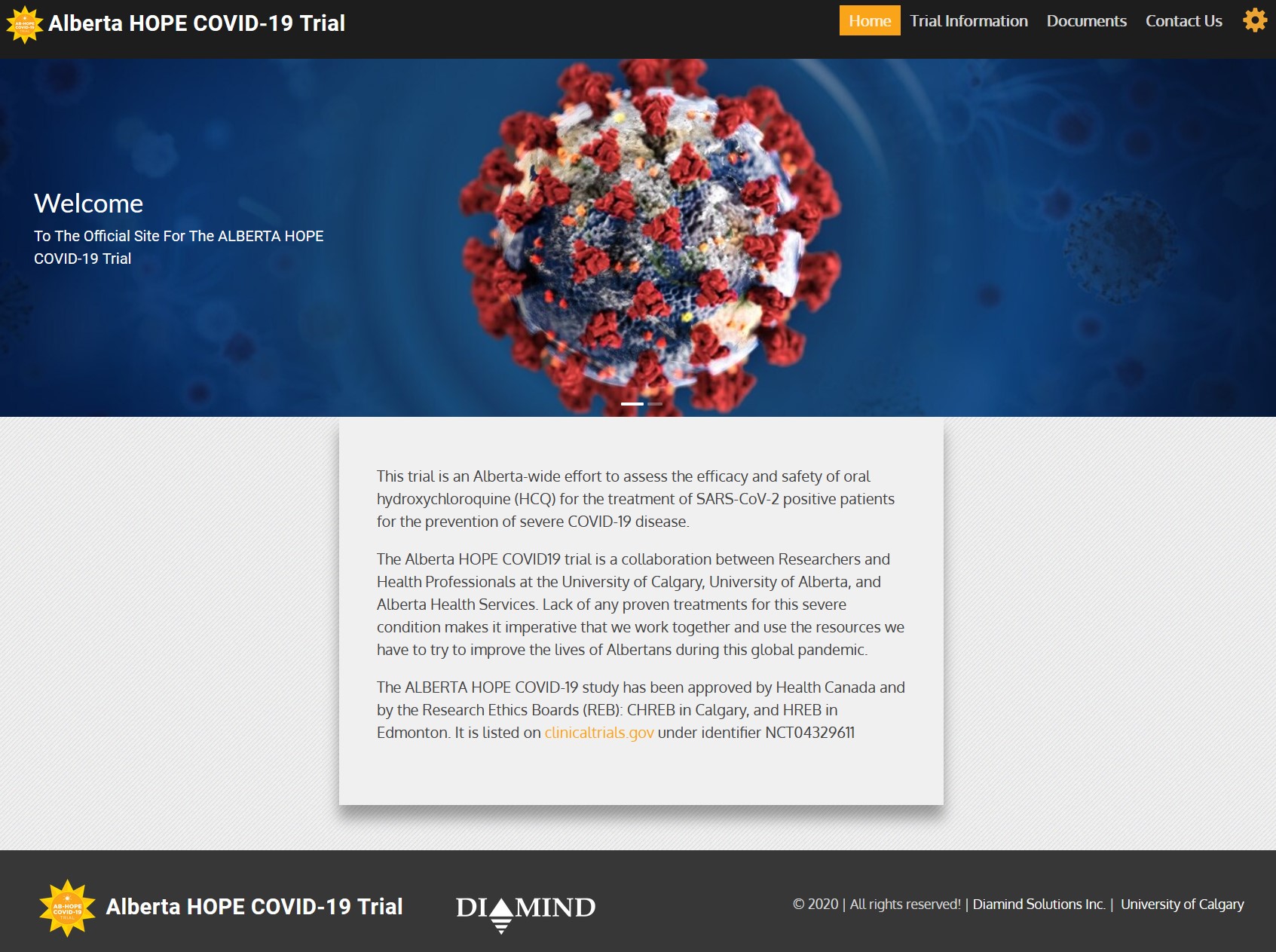
Alberta Hope COVID-19 Trial
The Alberta Hope COVID-19 Trial is a province-wide lead by researchers at the University of Calgary and the University of Edmonton to investigate the effectiveness of the well-tolerated drug hydroxychloroquine (HCQ) as an early intervention for Albertans who test positive for COVID-19.
Read Use CaseClinical Trial / Custom Application
AcT QuICR Trial
The AcT trial is a Pragmatic Phase III randomized open-label registry-based trial with blinded end-point assessment. The trial seeks to test whether intravenous tenecteplase can replace intravenous alteplase in patients who are otherwise eligible to receive the latter in routine care.
Read Use CaseClinical Trial / Custom Application
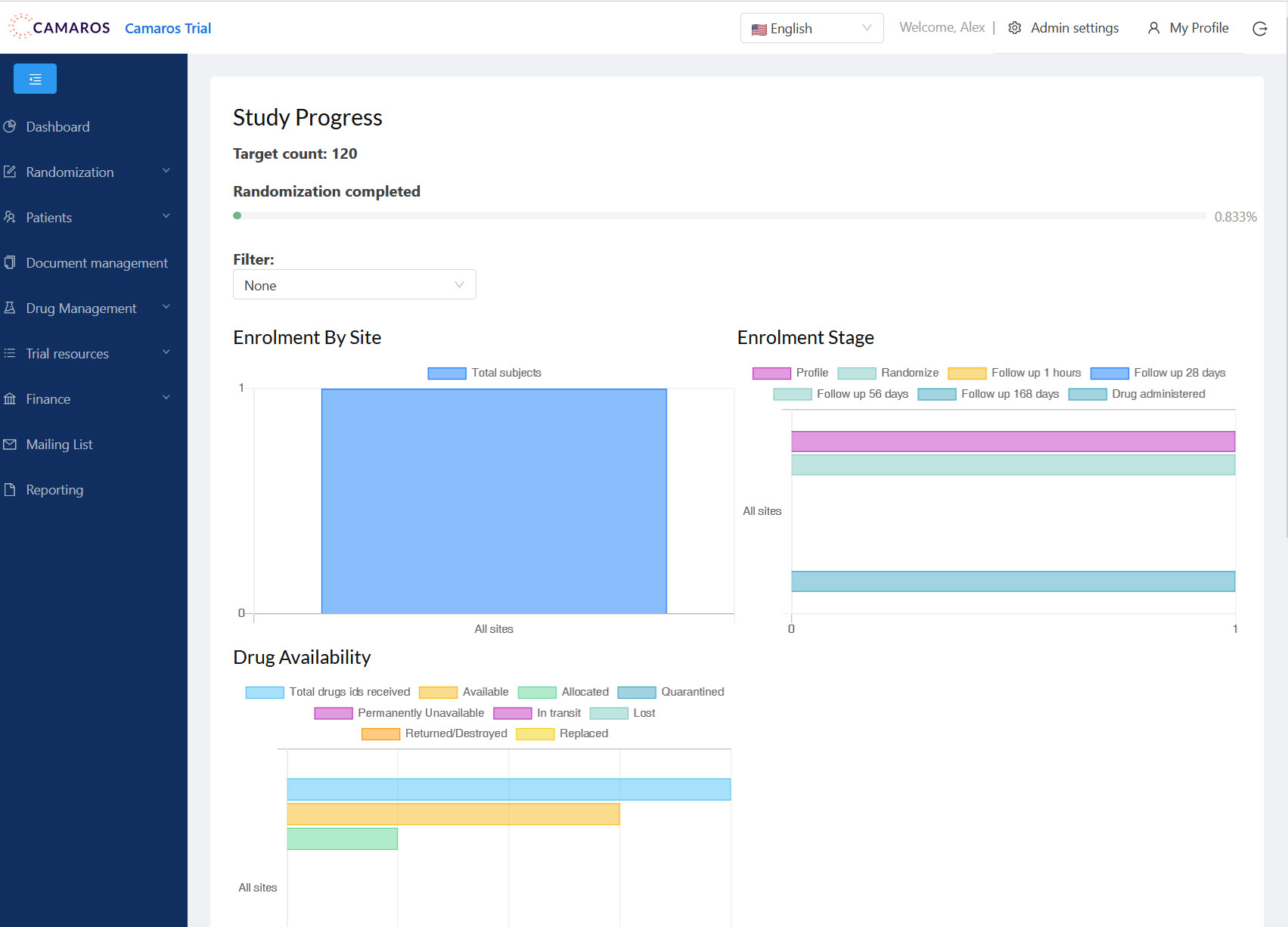
CAMAROS Trial
The CAMAROS trial is a multicentre, randomized, double-blinded, controlled phase II trial analyzing the effect of coupling a C-C chemokine receptor 5 (CCR5) antagonist, Maraviroc (Celsentri), and exercise to improve both upper and lower extremity recovery after a stroke.
Read Use CaseClinical Trial / Custom Application
ESCAPE-MeVO Trial
ESCAPE-MeVO is a multicenter, prospective, randomized, open- label study with blinded endpoint evaluation (PROBE design). Participants will be randomized to routine best medical stroke care governed by current guidelines (control group) or to EVT plus best medical care.
Read Use CaseResearch Platform / Custom Application
Collavidence Research Platform
Collavidence (Let's Get Proof) is a custom developed Research Platform that enables researchers to discuss and seek funding for their research initiatives. The platform brings together elements of crowd funding (e.g., KickStarter) with Reddit-style discussion groups to help improve medical research.
Read Use Case
Start Building
We're here to help
Depending on your needs and business plan, this may be a Clinical Trial Management Solution, a Patient Registry, a Health Data Hub, a Business Intelligence & Artificial Intelligence Solution, or even all of the above. We will meet with you, understand your needs, and then find the solution that offers the best fit for your needs!