Solutions
Explore our comprehensive solutions for clinical trials, registries, health data hubs, and platform features tailored to enhance research efficiency.
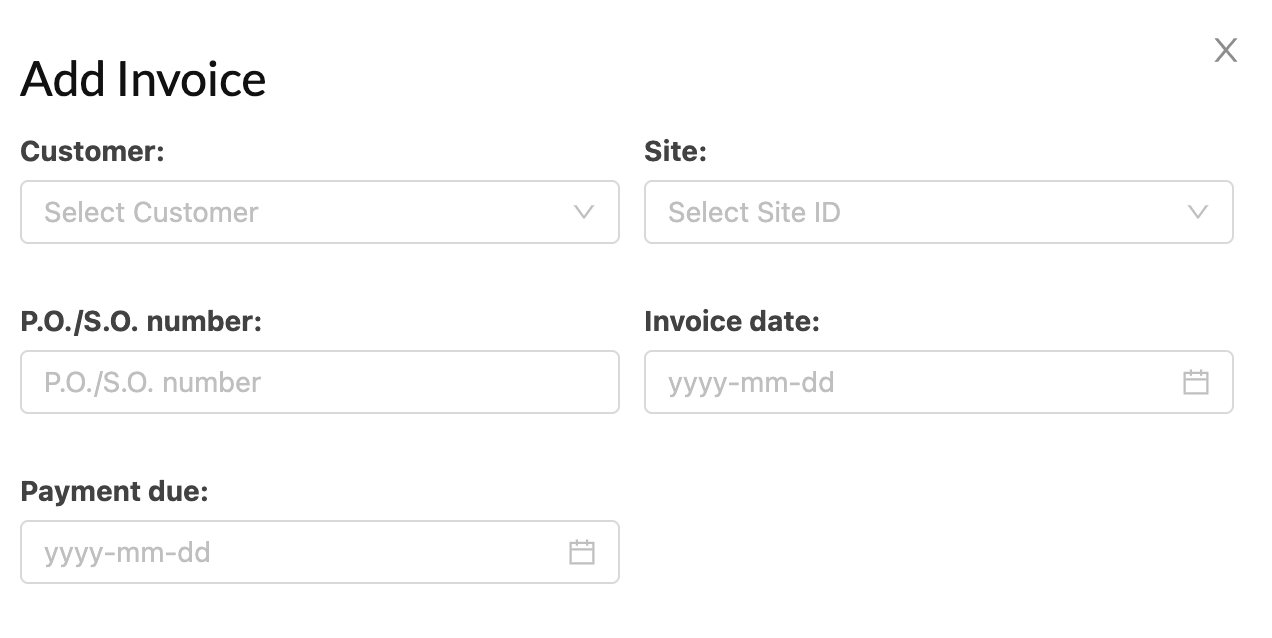
Clinical Trial Management Solutions
Innovative solutions to enhance clinical trial processes, from patient enrollment to adverse event management
Clinical Trial Management Solutions
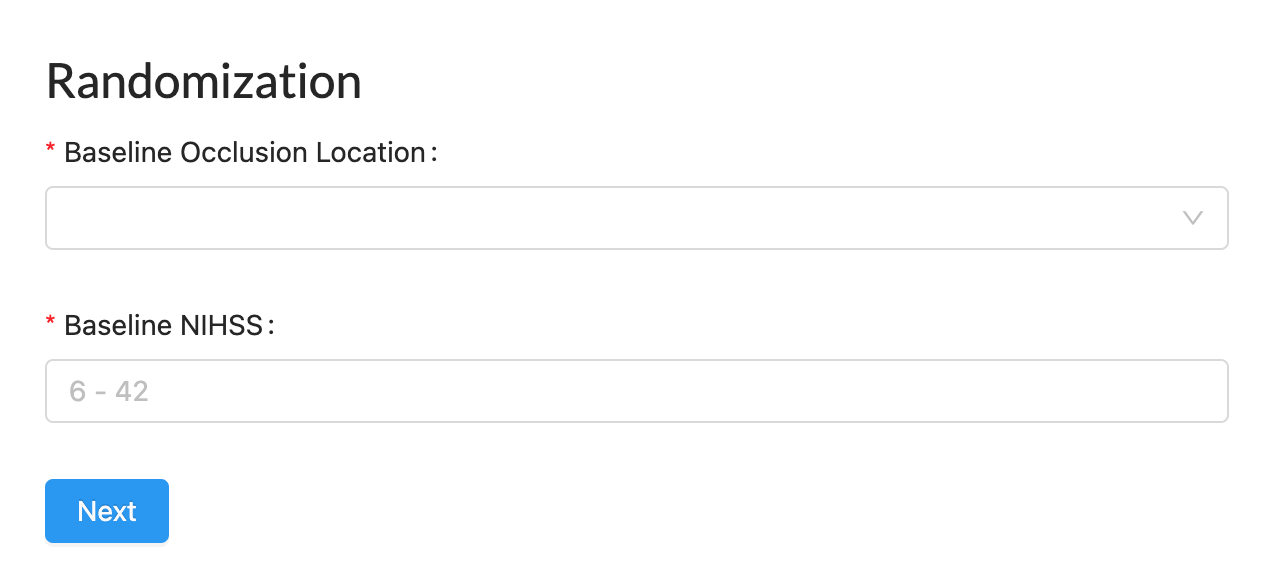
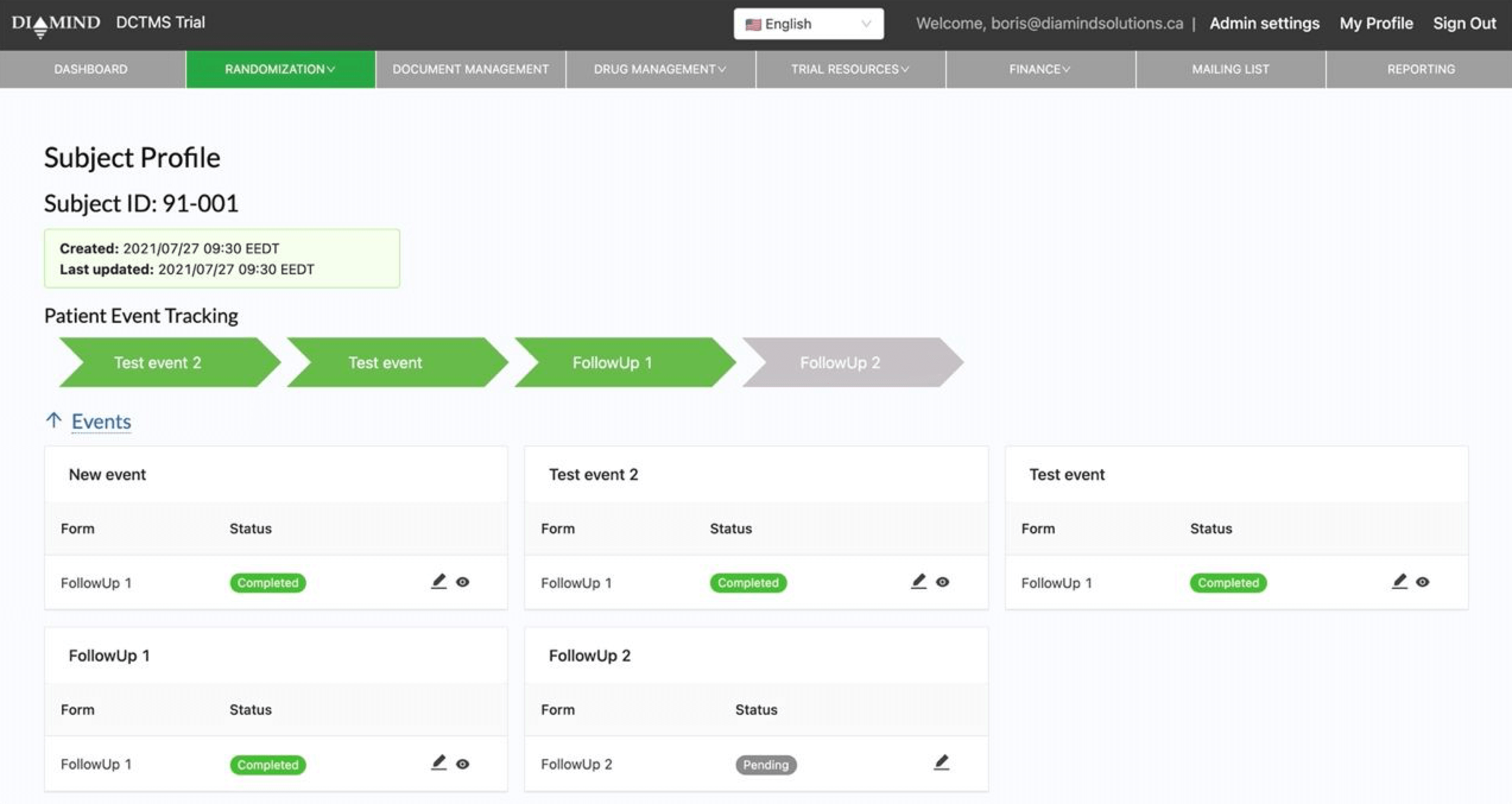
Randomization (IRT)
Adaptive and non-adaptive algorithms for improved enrollment
Read Use CaseClinical Trial Management Solutions
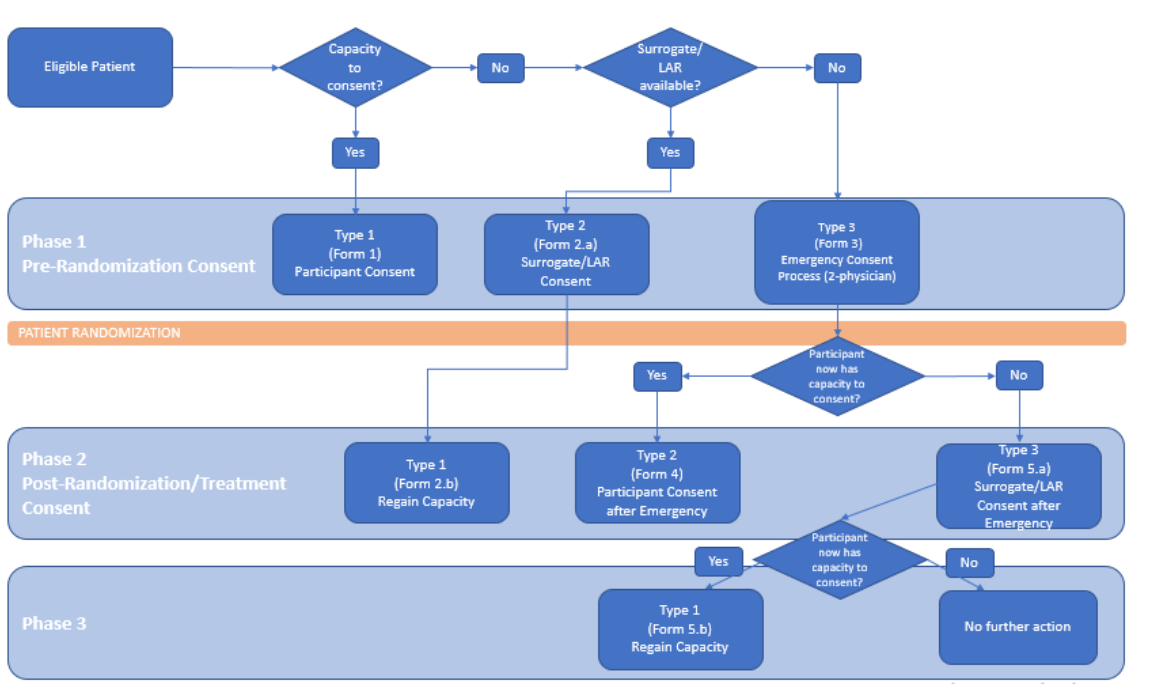
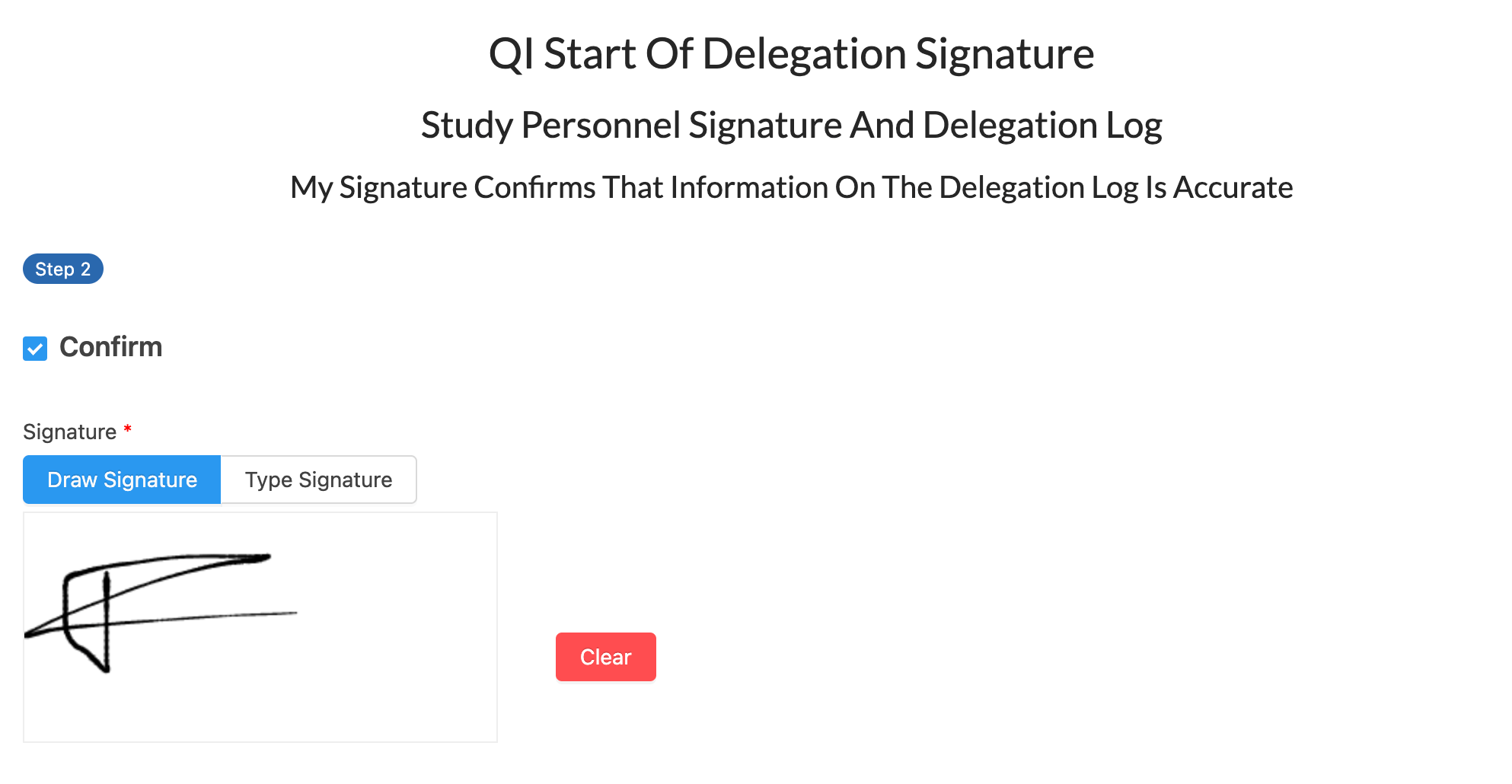
eConsent
Multi-workflow, multi-modality, multi-language consent
Read Use CaseClinical Trial Management Solutions
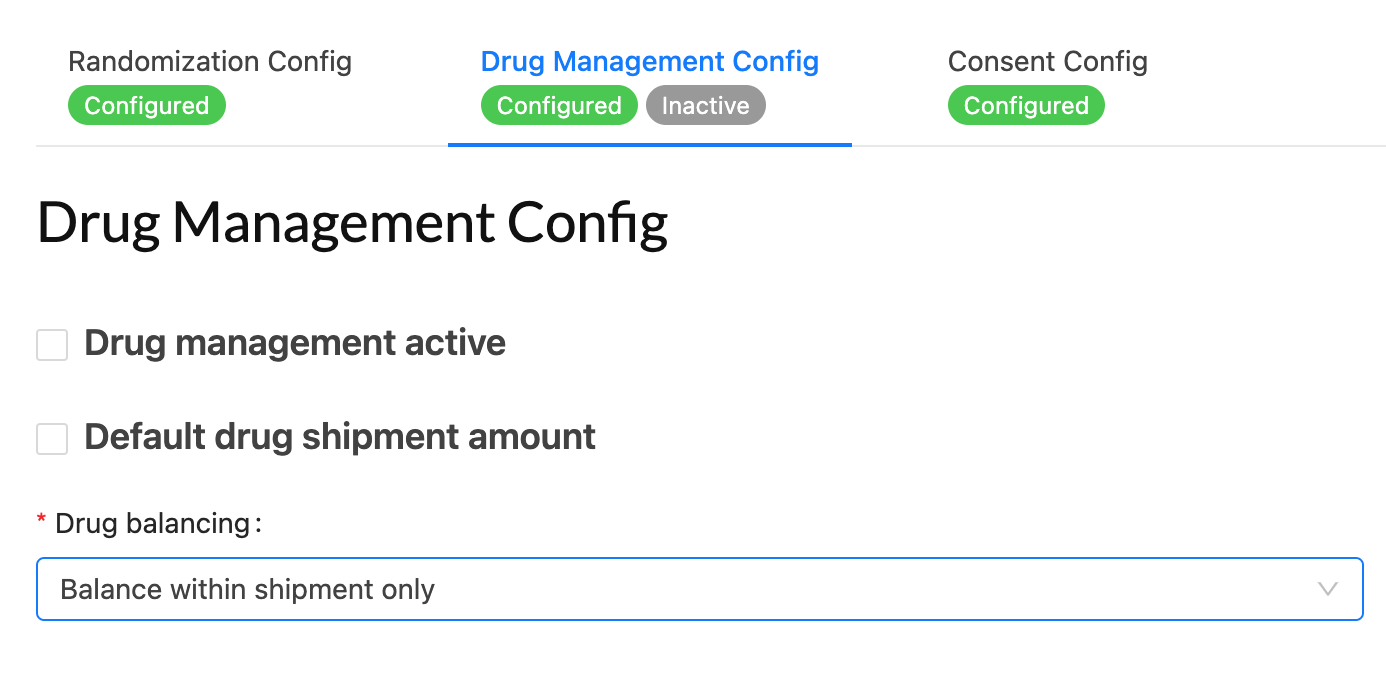
Drug/Device/IP Management
Comprehensive drug/device/IP allocation, shipping and tracking
Read Use CaseClinical Trial Management Solutions
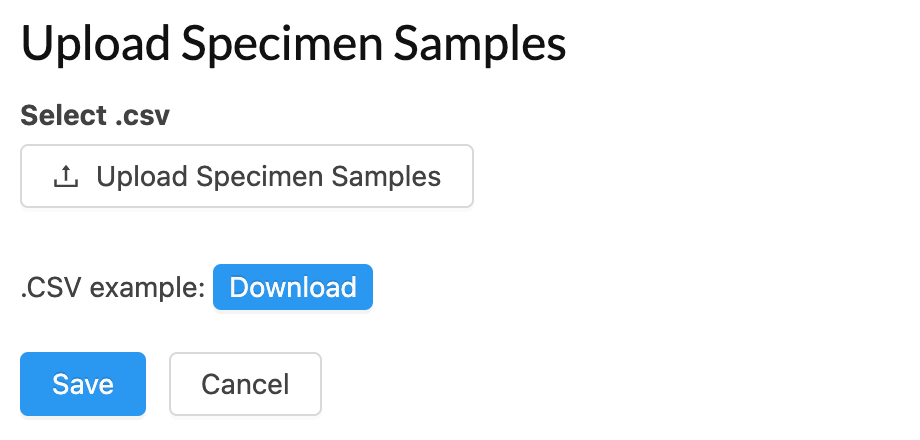
Bio-sample/Specimen Tracking
Integrated specimen collection, tracking and results management
Read Use CaseClinical Trial Management Solutions
Adverse Event Management
Collection, review and reporting of adverse events
Read Use CaseClinical Trial Management Solutions
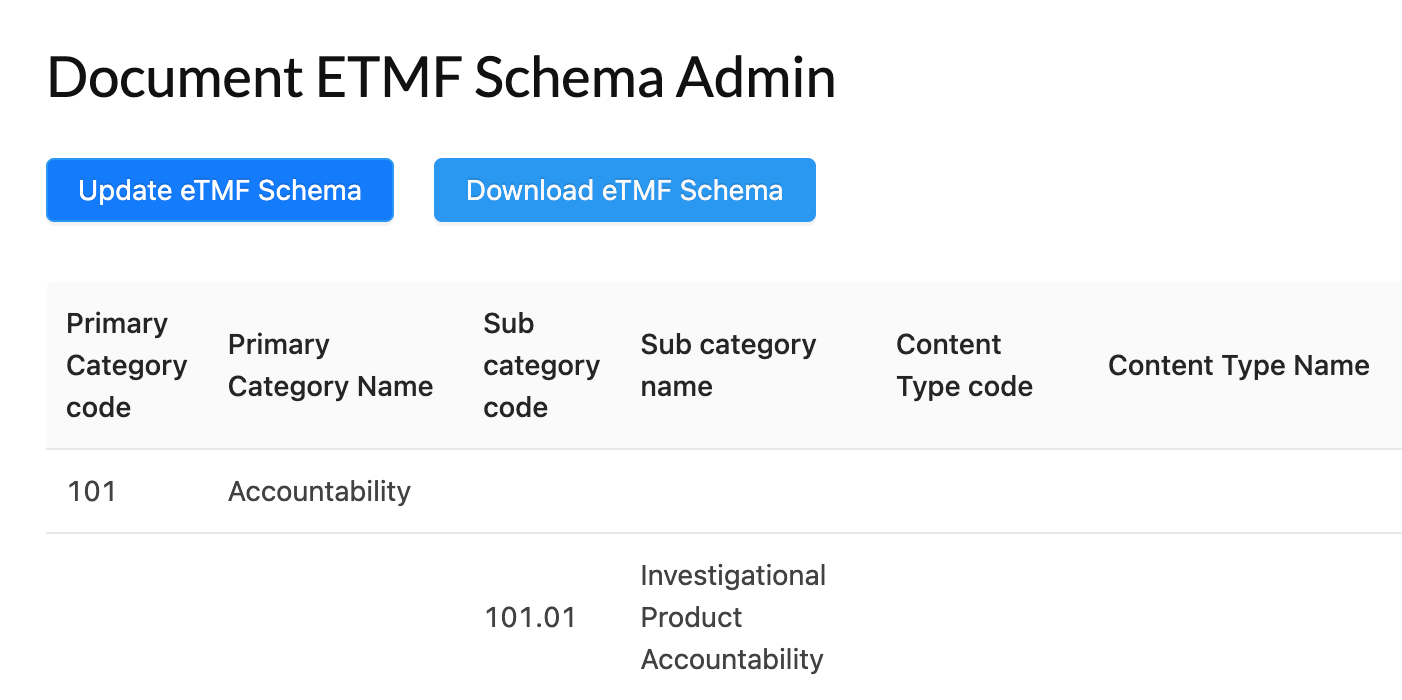
eTMF and Remote Monitoring
Electronic Trial Master File (eTMF) and electronic document management
Read Use CaseRegistry Solutions
Comprehensive tools for managing registries, patient visits, and related workflows
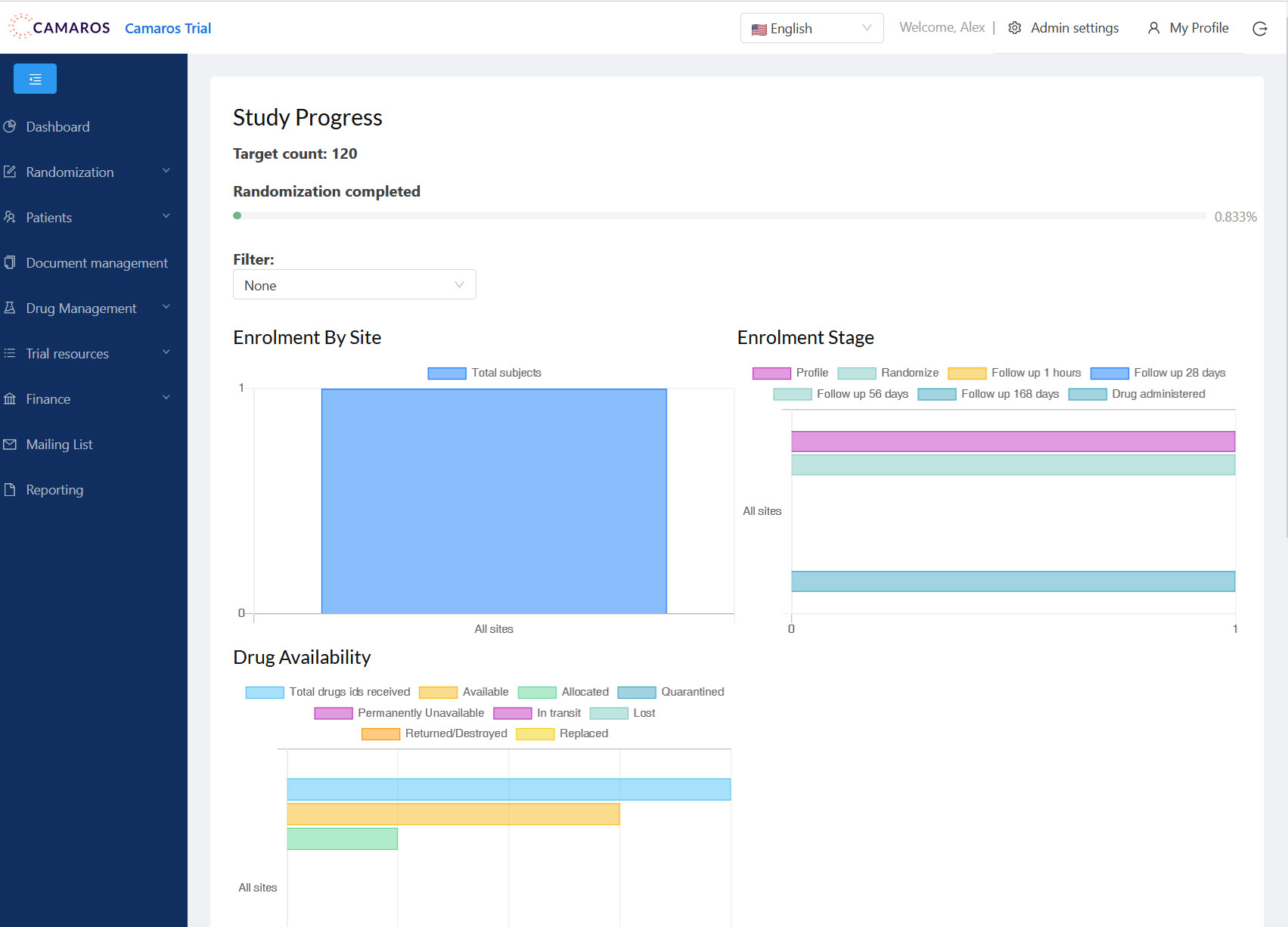
Health Data Hub Platform
An advanced platform for data management, integrations, and patient engagement
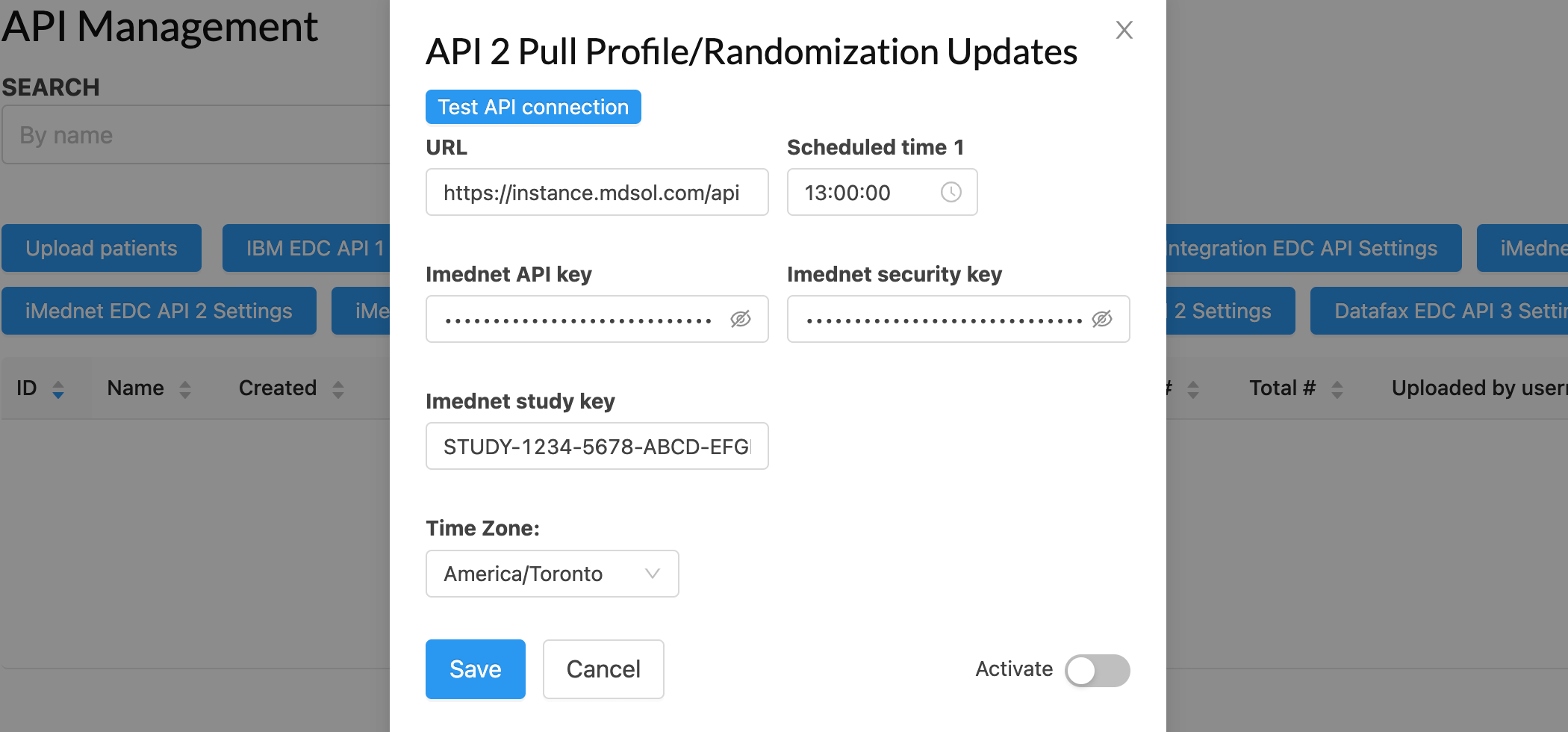
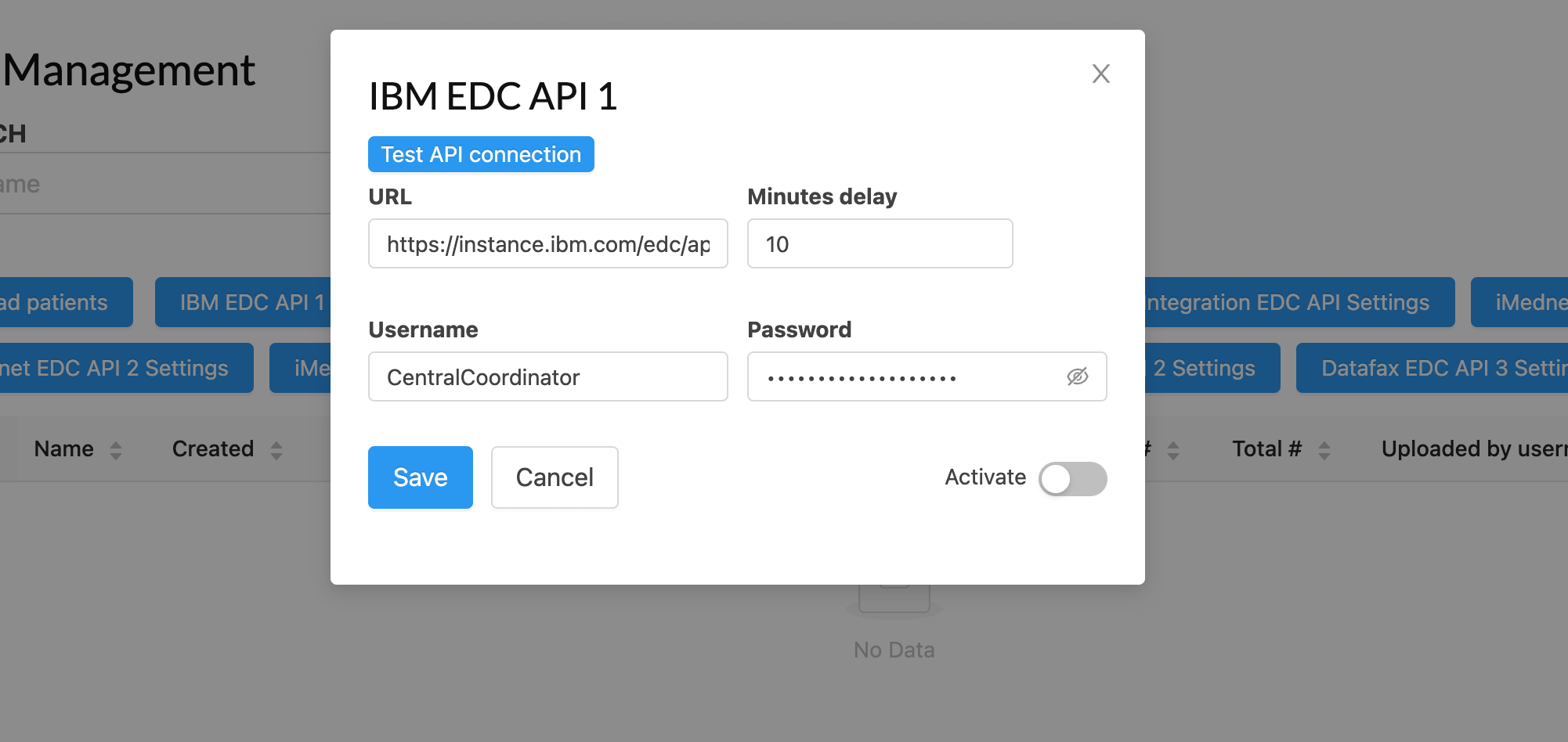
Common Platform Features
Core features for security, compliance, and efficient platform management
Common Platform Features
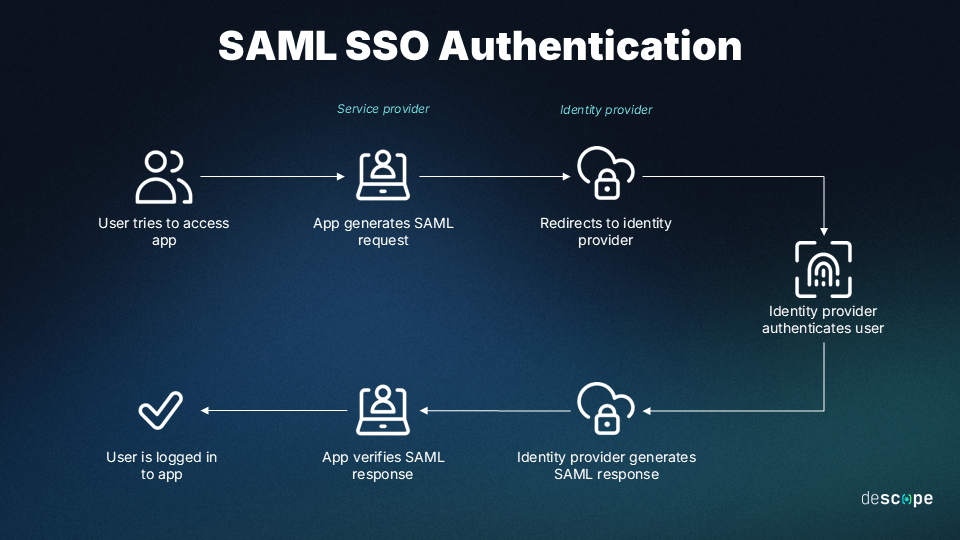
Identity Management
Manage authentication and authorization via your federated iDP
Read Use CaseCommon Platform Features
Training Resources
System and user-specific training resources for easier adoption
Read Use Case
Start Building
We're here to help
Depending on your needs and business plan, this may be a Clinical Trial Management Solution, a Patient Registry, a Health Data Hub, a Business Intelligence & Artificial Intelligence Solution, or even all of the above. We will meet with you, understand your needs, and then find the solution that offers the best fit for your needs!