ESACPE-MeVO Trial
ESCAPE-MeVO is a multicenter, prospective, randomized, open- label study with blinded endpoint evaluation (PROBE design). Participants will be randomized to routine best medical stroke care governed by current guidelines (control group) or to EVT plus best medical care.
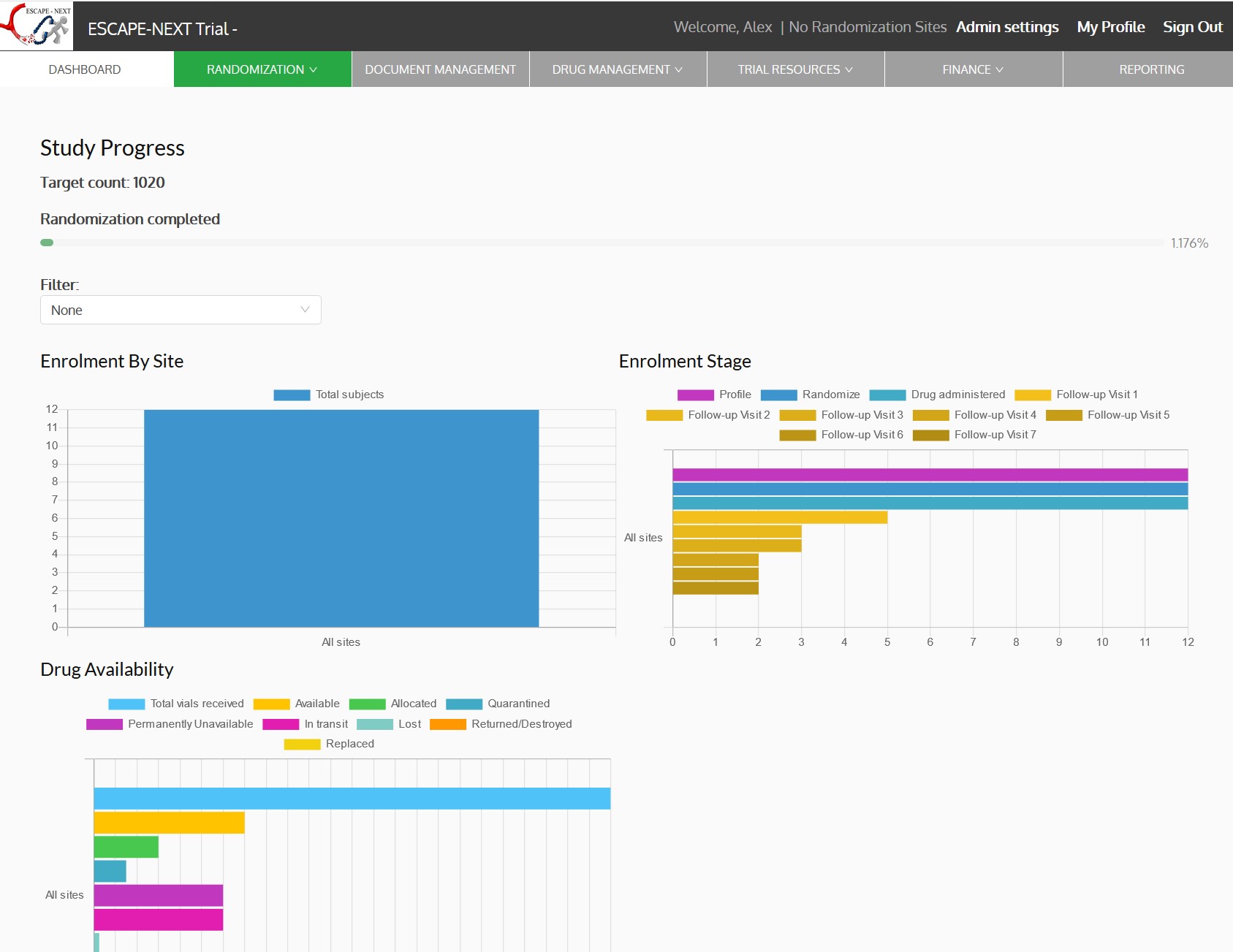
Stroke occurs when a blood clot causes a blockage in a blood vessel (artery) within the brain. This type of stroke is called an ischemic stroke and carries a high risk of disability or death. Stroke must be treated very fast. Any delay of even 10 minutes can result in the difference between an independent and a disabled outcome, and in some cases between life and death. Endovascular therapy (EVT) or Thrombectomy is a procedure to remove the blood clot (thrombus) from a blood vessel to reopen it (recanalization). Patients are likely to benefit from a thrombectomy procedure when it is performed in a larger blood vessel. Currently it is not known if thrombectomy procedure will benefit the patients presenting with the stroke that has been caused by a blood clot in a medium sized blood vessel (medium vessel occlusion, MeVO). The trial will enrol patients diagnosed with acute stroke due to a clot in the medium sized vessel. The patients will be randomized within 12 hours of their symptom onset to either standard of care or standard of care plus thrombectomy procedure. The participation will last for 12 months Escape MeVO coordinating centre is located at the University of Calgary. The trial will include approximately 50 sites and enroll 530 patients.
ESCAPE-MeVO is a custom designed Electronic Data Capture (EDC), Interactive Response Techonology (IRT) developed for the purpose of facilitating the data collection for the ESCAPE-MeVO Trial, while providing the following functions:
- Enables randomization of subjects based on a minimal sufficient balance algorithm
- Provides the ability to record and store patient profiles
- Provides eConsent capabilities for multiple modalities (patient, surrogate/SDM, emergency consent) via multiple workflows (electronic, paper, e-mail)
- Enabled complete electronic Trial Master File (eTMF) capabilities via our Document Management module
- Provides functionality for electronically managing and signing the Delegation of Authority log via a specialized electronic signature and custom workflow engine
- Dashboards, charts and reports
- Secure & Scalable
Want to know more about DCTMS? Please visit our DCTMS Product page or contact us for a free demo!
Other Links:
Ready to enroll?Get started today!
Our main areas of expertise are in the Healthcare, Academic, Education as well as the Energy Sector, where we provide solutions for a number of needs:
- - Clinical Trial Management Solutions (CTMS)
- - Interactive Response Technologies (IRT)
- - Randomization & Drug Management
- - Document Management & eTMF
- - eConsent
- - EHR Integrations
- - Custom Application Development
- - Business Intelligence & Data Integration