Patient Visit / Event Management: Streamlining Trial Milestones
Managing patient visits and study-related events is essential for maintaining trial schedules, ensuring compliance, and capturing accurate data. The Patient Visit/Event Management module in the Diamind Clinical Trials Management Solution (DCTMS) provides a centralized, customizable platform to plan, monitor, and manage patient interactions throughout the study lifecycle.
Key Features
- Custom Visit Schedules:
- Define milestones and visit timelines tailored to protocol requirements.
- Automate schedule generation based on patient enrollment and randomization.
- Integrated Data Collection and APIs:
- Capture visit-specific data using dynamic forms linked to patient records.
- Synchronize visit data with other trial modules, including randomization and drug/device management.
- Ability to push and pull data from other trial systems (e.g., DfDiscover, REDCap, iMedNet, IBM eClinical OS)
- Event Triggers and Notifications:
- Automate reminders and notifications for upcoming visits or overdue events.
- Trigger related processes, such as specimen collection or adverse event reporting.
- Real-Time Monitoring:
- Track visit completion and adherence to protocol-defined timelines.
- Gain actionable insights with dashboards that monitor patient progress and site performance.
- Compliance Support:
- Ensure visits align with regulatory requirements through detailed audit trails.
Description
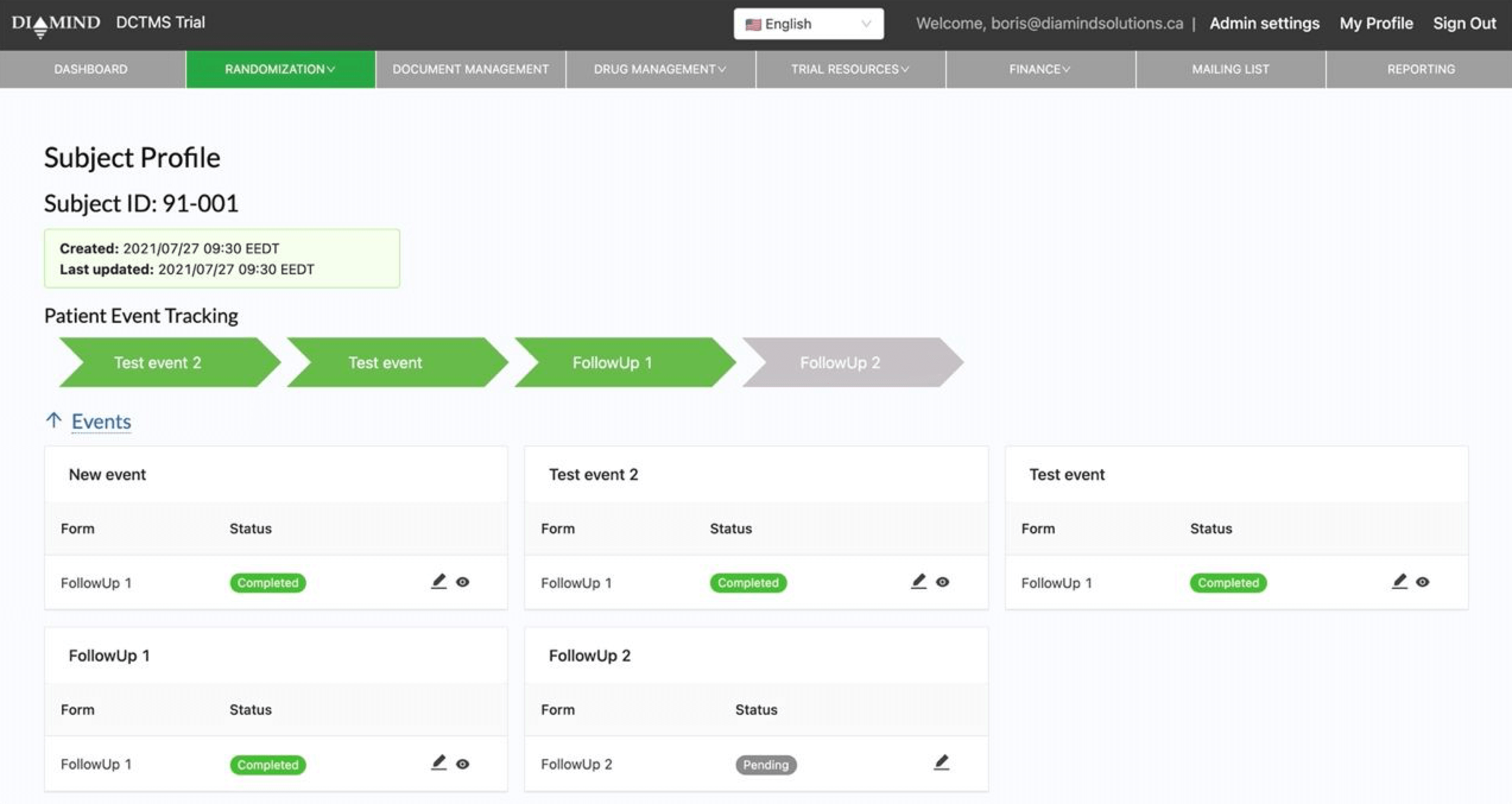
One of the most important components post patient enrollment is to monitor the progression and outcome of the treatment via several visits and assessments. Traditionally done via paper Case Report Forms (CRFs), this method has a number of issues as these forms are often difficult to locate for large trials and even more difficult to centralize for the final analysis.
While some systems have been offering electronic-CRFs (e-CRFs) over the past years as part of Electronic Data Capture (EDC) capabilities, most of these systems were developed with paper and fax technologies in mind and were not modernized for fully web-enabled technologies (e.g., most solutions still require software clients to be installed on hospital computers and do not support table or mobile data capture).
Additionally, these solutions lack the ability to generate visit schedules based on randomization parameters, as well as lack integration with the rest of a trial’s systems (e.g., randomization, consent, drug management).
This leads to a significant amount of lost time with dual data-entry, as well as non-compliance with patient visit schedule.
Last, in scenarios when trials or groups already use an EDC, there is a strong desire to integrate data and workflows with the EDC so that data accuracy is preserved and dual data entry becomes a thing of the past.
Through our API module and patient modules, we have developed a solution that can synchronize data with existing EDCs, while enhancing some of the capabilities of these systems. For example, you can now have our system automatically generate a patient’s visit schedule, group visits into milestones for event tracking and site payments, as well as make some of this information available in the patient’s portal.
How It Works
- Scheduling and Automation:
- Build visit schedules and milestones tailored to study protocols, with automated adjustments for randomization or specific cohorts.
- Visit Management:
- Capture all data associated with patient visits, including clinical assessments, forms, and observations, directly in the system.
- Event Triggers:
- Automate linked events like specimen collection, eConsent updates, or drug allocation, minimizing manual processes.
- Dashboards and Analytics:
- Monitor visit adherence and patient progression, ensuring protocols are followed and data collection is complete.
Why Choose Our Patient Visit/Event Management Module?
The Patient Visit/Event Management module simplifies the complexities of visit tracking by automating schedules, linking data collection to trial events, and providing real-time insights. Its integration with other DCTMS modules ensures that all trial activities are seamlessly coordinated, reducing administrative burden and improving data accuracy.
Driving Patient-Centric Research
This module is designed to enhance trial efficiency while maintaining patient-centricity. By ensuring visits are well-organized, data is accurately captured, and schedules are adhered to, it supports high-quality trial outcomes. Its scalability and adaptability make it suitable for trials of all sizes and complexities.
Streamline Your Trial Milestones
Contact us today to learn how the Patient Visit/Event Management module can enhance your clinical trial workflows and ensure protocol compliance.