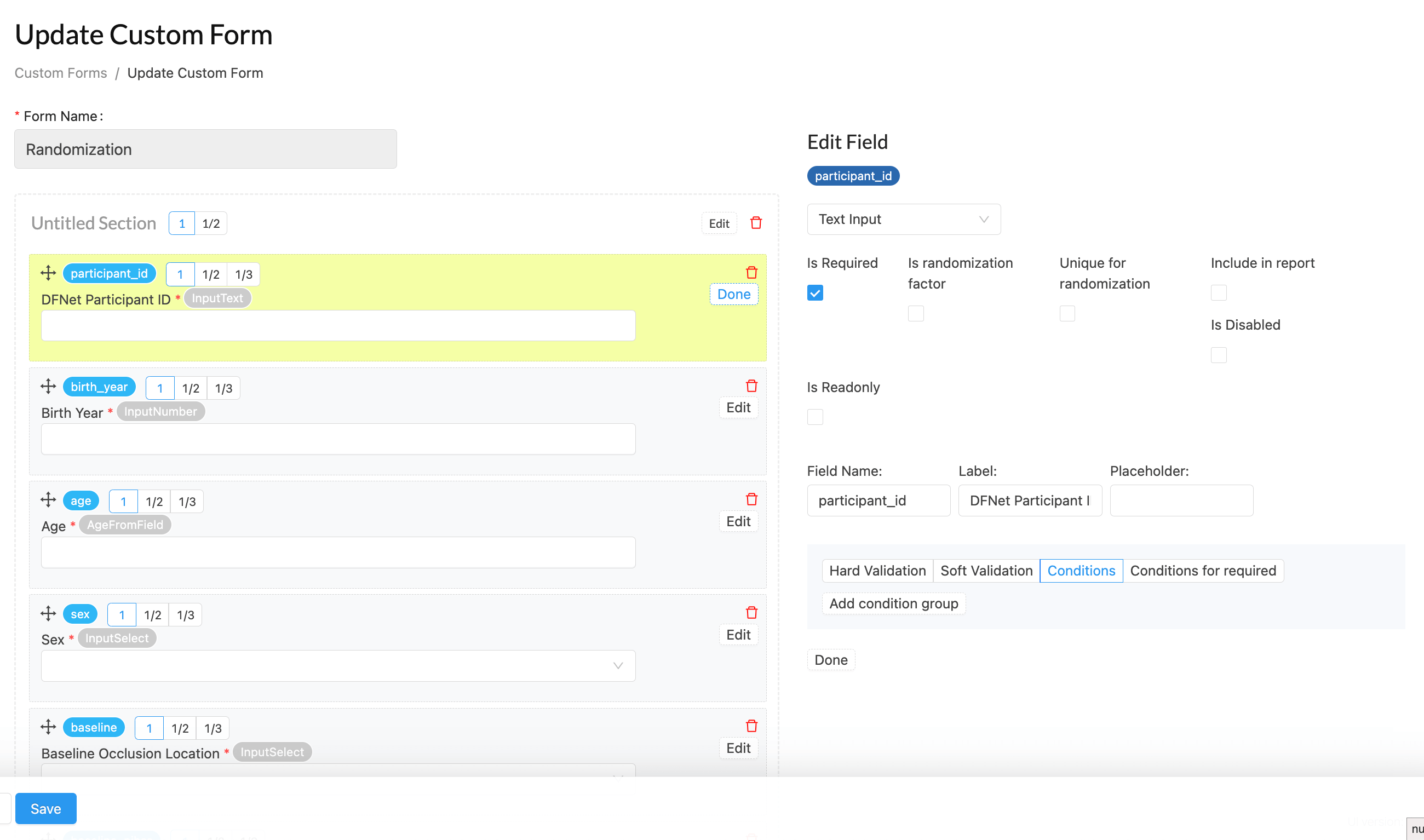
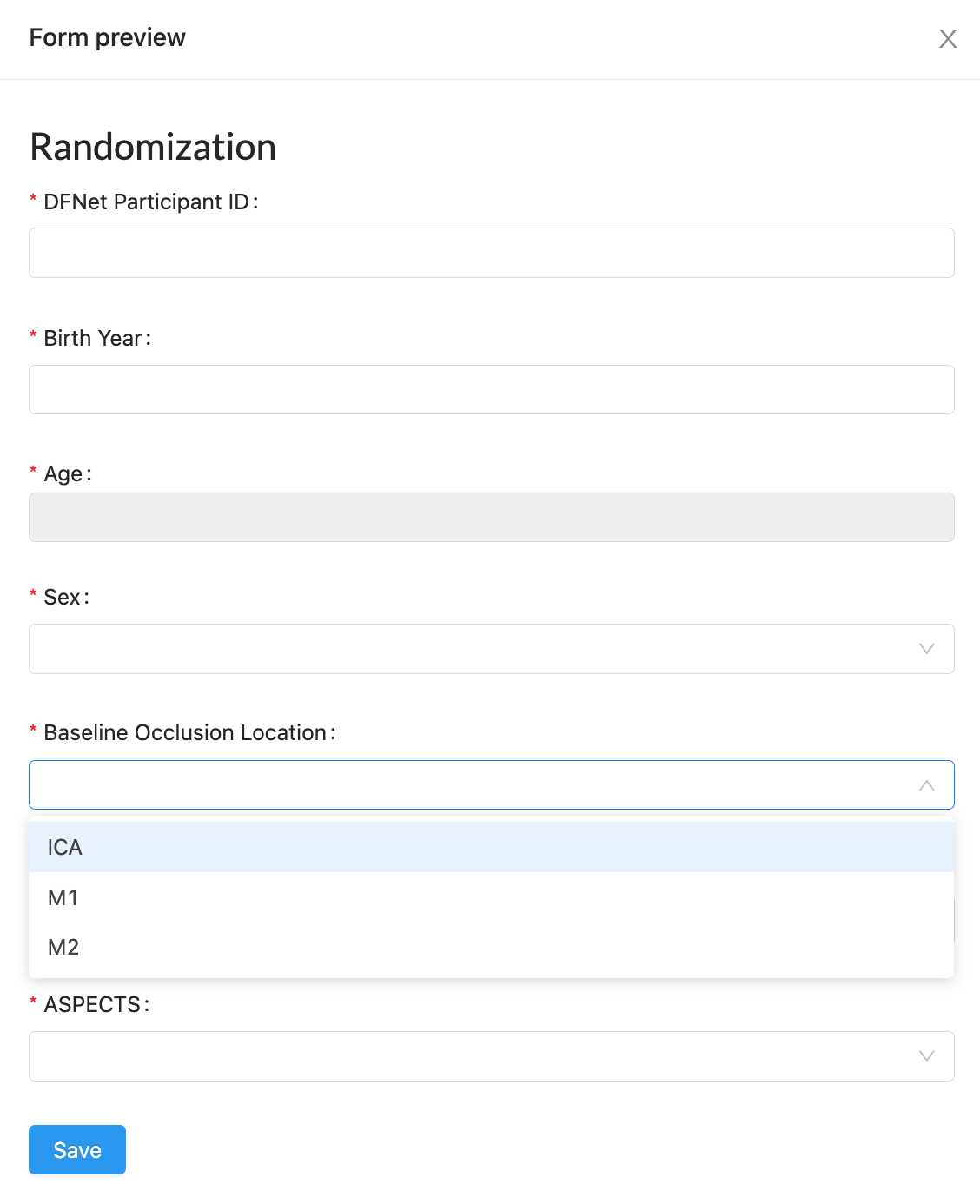
CRF / Form Builder: Customizable and Integrated Data Capture
Accurate and efficient data collection is the backbone of successful clinical trials. The CRF/Form Builder module in the Diamind Clinical Trials Management Solution (DCTMS) empowers researchers to create, manage, and integrate custom case report forms (CRFs) and data collection tools tailored to the unique needs of their studies. This module simplifies data capture and ensures seamless integration with other trial processes, improving both efficiency and data quality.
Key Features
- Custom Form Creation:
- Build forms tailored to specific study protocols and data requirements.
- Support for various data types, including numerical, categorical, text, and date fields.
- Dynamic Workflows:
- Link forms to patient visits, events, and milestones for automated data capture workflows.
- Configure conditional logic to adapt forms based on study-specific rules and inputs.
- Integration with Trial Systems:
- Synchronize form data with other DCTMS modules, including randomization, eConsent, and drug management.
- Enable data exchange with external EDC and other systems via API integrations.
- Real-Time Data Validation:
- Incorporate validation rules to minimize data entry errors and ensure consistency.
- Generate alerts for incomplete or invalid data entries.
- User-Friendly Interface:
- Drag-and-drop form builder for rapid form creation and updates.
- Role-based access controls to manage form editing and data entry permissions.
Description
The CRF/Form Builder module in the Diamind Clinical Trials Management Solution (DCTMS) is designed to streamline data collection and enhance the efficiency of clinical trial workflows. This module enables research teams to create fully customizable case report forms (CRFs) tailored to their study protocols. Its user-friendly drag-and-drop interface simplifies form creation, allowing teams to define fields, validation rules, and conditional logic without the need for technical expertise.
Seamlessly integrated with other DCTMS modules, the CRF/Form Builder ensures that data collected during patient visits, adverse event reporting, or other trial milestones is automatically synchronized across the platform. Researchers can attach forms to specific trial events, enabling automated workflows that reduce manual errors and administrative burden. Built-in data validation rules help maintain accuracy and consistency, flagging incomplete or invalid entries in real-time to ensure data quality.
This module is adaptable to trials of any size or complexity, supporting various data types and multi-language capabilities. By integrating data capture with trial processes, it eliminates silos and enables a holistic view of trial progress. Whether used for simple forms or intricate multi-step workflows, the CRF/Form Builder module provides the tools needed to manage data efficiently, empowering researchers to focus on delivering actionable insights and achieving trial objectives.
How It Works
- Form Design:
- Use the intuitive drag-and-drop interface to design forms customized to your study needs, including multi-language support.
- Workflow Integration:
- Attach forms to specific study events, such as patient visits or adverse event reporting, ensuring streamlined data collection.
- Real-Time Data Capture:
- Collect and validate data in real-time through user-friendly electronic interfaces accessible on desktop or mobile devices.
- Data Synchronization:
- Automatically integrate captured data into the central trial database, minimizing manual errors and redundancy.
Why Choose Our CRF/Form Builder Module?
The CRF/Form Builder module enables study teams to take control of data collection with unparalleled flexibility and ease. Its seamless integration with other DCTMS modules eliminates data silos and ensures a holistic approach to trial management. By automating data capture workflows and incorporating real-time validation, it reduces administrative burden and improves the accuracy and reliability of collected data.
Enhancing Data-Driven Research
From simple forms to complex, multi-step workflows, this module supports trials of all sizes and complexities. It ensures that data capture aligns with regulatory standards and study protocols, empowering teams to focus on insights and outcomes rather than manual processes.
Enhance Your Data Collection
Contact us today to learn how the CRF/Form Builder module can streamline your clinical trial workflows and elevate your research capabilities.